Model of Navier-Stokes/Korteweg (NSK)
van der Waals fluids
Model of Navier-Stokes/Korteweg
The mathematical model is based on the low Mach formulation of the Navier-Stokes equations. The mass balance writes
The impulsion balance equation makes appear the pressure tensor
where \(\overline{\overline{\boldsymbol{P}}}\) is the pressure tensor which is defined by
In Eq. (79) \(p^{eos}(\rho,T)\) is the thermodynamic pressure defined from the potential by:
The thermodynamic pressure is a function of density \(\rho\) and temperature \(T\).
Equations of state (EoS)
Several Equations of State (EoS) exist in the literature to simulate two-phase flows: Van der Waals, Carnahan-Starling, Peng-Robinson, Redlich-Kwong, etc. Only the first two have been tested in LBM_Saclay.
Van der Waals EoS
The Van der Waal EoS writes
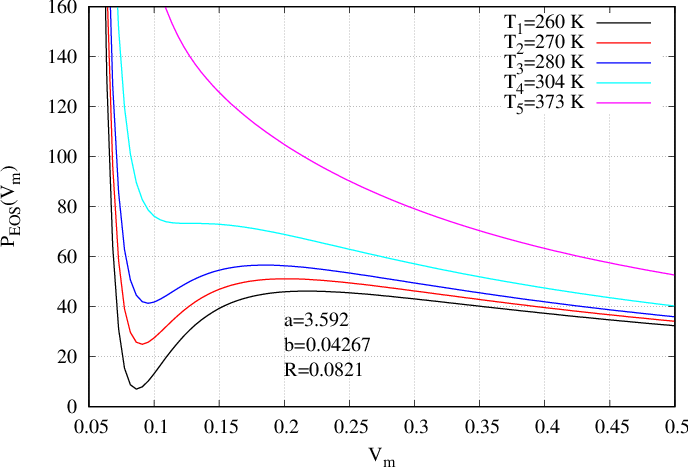
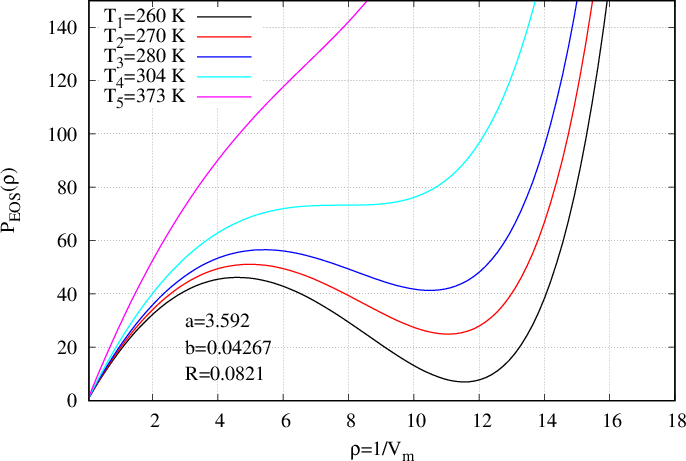
where \(T_k\) is a constant temperature which must be indicated in the input file as well as the two coefficients \(a,b\) and the constant of perfect gas \(R\). Such an equation of state is presented in Fig. 38 for five different temperatures (subscript \(k=1,...,5\)). That EoS can be derived from a thermodynamic potential (94) where \(\mathcal{W}_{vdW}\) is defined by
Finally the chemical potential \(\mu_{\rho}\) is derived by the Euler-Lagrange equation \(\mu_{\rho}=\mathcal{W}^{\prime}(\rho)-\kappa\boldsymbol{\nabla}^{2}\rho\):
On Fig. 37 the pressure is presented as a function of volume \(V\) whereas on Fig. 38 it is presented as a function of density \(\rho\). On those figures, one temperature \(T_5\) (the magenta curve) is above the critical temperature. The curve is monotonous and only one density corresponds to one pressure. For temperature \(T_4\) (cyan curve) there is an inflexion point corresponding to the critical temperature (zero derivative with respect to rho). At last, for three next temperatures \(T_1,T_2,T_3\) (respectively black, red and blue curves), the pressure presents a cubic form enabling the existence of two densities corresponding to one pressure.
Carnahan-Starling EoS
One improvement of the vdW EoS is the Carnahan-Starling EoS which writes:
where, once again, the input parameters are \(R,T,a,b\). That EoS can be derived from the potential:
Finally, the chemical potential writes:
Two other popular EoS exist in the literature. We only mention them here. They could be tested in the future in LBM_Saclay.
Redlich-Kwong (RK) eos
Peng-Robinson (PR) eos
Alternative forms of tensor pressure
In most of numerical methods that implement the NSK model, the pressure tensor Eq. (79) is not directly discretized because there exist two equivalent algebraic forms which are easier to implement. The first one is the potential form and the second involves the chemical potential.
First form: potential form
with
Demo:
Second form: chemical potential form
with
Demo
Section author: Alain Cartalade